orange book pharmacy ab rating
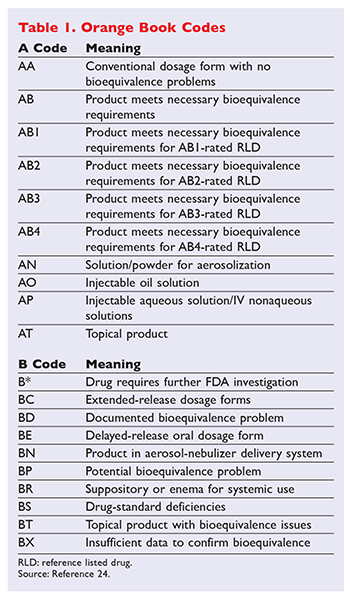
Every drug listed in the Orange Book has a 2-letter code. Log in Sign up.
If each inhaler contains 200 puffs what is the days supply of 2 inhalers.

. The Orange Book is a reference source that gives insight on whether or not two drugs have Therapeutic Equivalences. Start studying Pharmacy Tech Study. As such it is essential that pharmacists practicing in New York State have a thorough understanding of the Orange Book and the leading role it plays in ensuring the safety and effectiveness of the drug products dispensed in.
Roseman University of Health Sciences College of Pharmacy South Jordan USA. THIS SET IS OFTEN IN FOLDERS WITH. As pharmacists are aware in recent weeks the Food and Drug Administration FDA changed the Orange Book equivalency rating of extended release methylphenidate products manufactured by Mallinkrodt and Kudco from AB to BX due to concerns about bioavailability equivalency with Janssen Pharmaceuticals Concerta product.
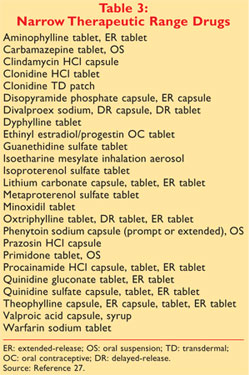
Recently filed suit in the US. An A designation means that the FDA considers the drug to be the therapeutic. Pharmacists should be aware that the Narrow Therapeutic Index Drug concept is found in the states Product Selection Law GS.
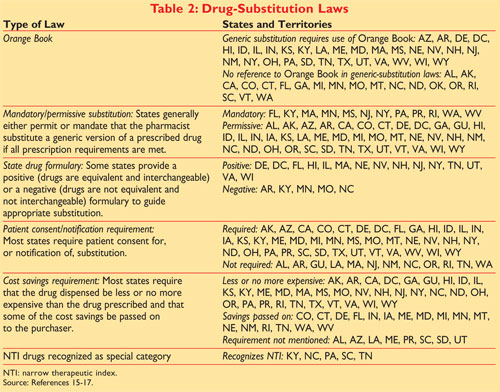
FDAs orange book and ab ratings of pharmaceutical drug products. In The Orange Book the letter AB rating indicates that products. Generic interchange has become routine in pharmacy practice.
For drugs that are off patent the generic drug may be the only form available. FDAs orange book and ab ratings of pharmaceutical drug products. - Orange Book - Trissels - USP-NF.
- Orange Book - Substitute References - Pharmacy Drug Reference. It has come to the Boards attention that one manufacturer has received an AB Rating for their Levothyroxine product. A guide to community pharmacist.
Preface to 42nd Edition. However shorty after the launch of the generics the FDA received numerous complaints regarding the products. What rating must a generic have to be considered clinically bioequivalent to the brand name drug.
The first letter indicates that the FDA has either concluded a generic formulation is therapeutically equivalent to the reference drug an A Code rating or that the compared drugs. Search approved drug products by active ingredient proprietary name. Over 75 of filled prescriptions are done so with a generic formulation which has resulted in significant savings in healthcare costs.
Also the annual Orange Book Edition Appendices A B and C in PDF format are updated quarterly. According to the FDA two products are considered to be bioequivalent if the 90 clearance CI of the relative mean Cmax AUC 0 - t and AUC 0 - of the generic drug to the brand - name. Orange Book in choosing drugs for generic substitution.
Applying the Ratings Code to Antihypertensive Agents. When the first generic Concerta products came to market late in 2013 they were Orange Book AB rated to the brand name. A de o co aacs 55 o.
The Orange Book uses Therapeutic Equivalence codes TE codes a short series of letters and sometimes numbers eg AB AB2 BX to categorize drugs based upon their assessed equivalency. The FDA approves new drugs or existing drugs for new uses following a series of double-blind randomized clinical trialsEarly phases of this process involve tests. As oae boo ad ab as o aaceca d odcs.
This means they were classified as bioequivalent by the FDA and therefore were substitutable with one another. The publication Approved Drug Products With Therapeutic Equivalence Evaluations the List commonly known as the Orange Book identifies drug products approved on the. District Court for the District of Columbia alleging that Astra Zeneca AZ illegally monopolized the market for its proton pump inhibitor drugs Prilosec Nexium and their AB-rated generic equivalents by engaging in a scheme to convert the prescription drug market for.
The electronic Orange Book provides guidance that is beneficial for pharmacy personnel to review. A patient has a script for Ventolin HFA 2 puffs po QID prn. Rucha Pathak Roll No.
The first letter -- A or B -- indicates whether the drug is therapeutically equivalent to other pharmaceutically equivalent products. Learn vocabulary terms and more with flashcards games and other study tools. Understanding the Orange Book.
Search the Orange Book Database. 1015406mojbb20150100013 Table 1 Summary of FDAs Orange Book Therapeutic Equivalence Codes Code Interpretation. 2 votes and 4 comments so far on Reddit.
1 Need of the Orange Book Definition Introduction to 2 History 3 the Orange Book Objectives 4 3 Contents of the Orange Book 5 18 4 Cumulative Supplement 19 5. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring MD 20993 1-888-INFO-FDA 1-888-463-6332 Contact FDA. Pharmacy Tech Test 2.
To limit costs many doctors write prescriptions for generic drugs whenever possible. A guide to community pharmacist. Drugs rated as AB1 are bioequivalent and pharmaceutically equivalent to each other as are drugs that are AB2-rated and so on.
Basics in drug approval process with reference to the Orange Book Presented by. Vijay M Kale Assistant Professor of Pharmaceutical Sciences Roseman University of Health Sciences College of Pharmacy 10920 S Riverfront Parkway. Levothyroxine qualifies as an NTI drug under this statute which means that.
- AB rating - DAW code - the meds manufacturer - the meds inactive ingredients. According to the Orange Book A codes denote drug products that are considered to be therapeutically equivalent to other pharmaceutically equivalent products 4 On the other hand a B designation signifies drug products that FDA at this time considers not to be therapeutically equivalent to other pharmaceutically equivalent products 4 The second. A group of pharmacies led by Walgreen Co.
Theoretically any generic drug that is bioequivalent to its brand-name counterpart may be interchanged with it. 1 The FDA regulates the approval of generic drugs and ensures that generic formulations are equivalent to their brand. For more information on the Orange Book including its history see the Orange Book Preface.

Investigational New Drug Orange Book Understanding On 505 B 2 A

Primary Assessment Abcde Emergency Nursing Health Assessment Nursing Nursing Tips
Insights Into Effective Generic Substitution

Investigational New Drug Orange Book Understanding On 505 B 2 A

Change Is The Law Of Nature Essay In 2021 Summary Writing College Application Essay Homework Help

Investigational New Drug Orange Book Understanding On 505 B 2 A

Electronic Orange Book Youtube

Investigational New Drug Orange Book Understanding On 505 B 2 A

Pin On It S A Boy And A Girl Twins

Investigational New Drug Orange Book Understanding On 505 B 2 A

Medications That Require Special Handling Ppt Video Online Download

Investigational New Drug Orange Book Understanding On 505 B 2 A